How To Make Gasoline
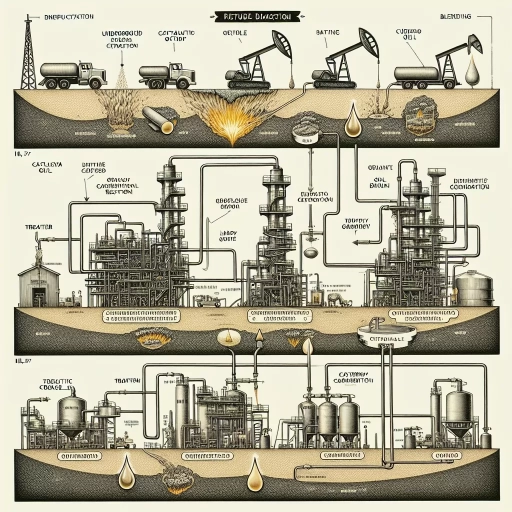
Gasoline is a vital component of modern life, powering vehicles and machinery that drive economies and societies forward. However, have you ever wondered how this essential fuel is made? The process of creating gasoline is complex and involves several stages, from understanding the basics of gasoline production to extracting and refining crude oil, and finally, converting it into the gasoline we use every day. In this article, we will delve into the world of gasoline production, exploring the intricacies of this process and providing a comprehensive guide on how to make gasoline. To begin, it is essential to understand the basics of gasoline production, including the types of crude oil used, the refining process, and the various additives that enhance the fuel's performance. By grasping these fundamental concepts, we can better appreciate the subsequent stages of gasoline production, including the extraction and refining of crude oil, and the conversion of crude oil into gasoline. Let's start by understanding the basics of gasoline production.
Understanding the Basics of Gasoline Production
Gasoline is a refined product derived from crude oil, and its production involves a complex process. To understand the basics of gasoline production, it is essential to delve into the crude oil refining process, the chemical composition of gasoline, and the various types of gasoline available. The crude oil refining process is a critical step in gasoline production, as it involves the separation of crude oil into various fractions, including gasoline. The chemical composition of gasoline is also crucial, as it determines the fuel's performance and environmental impact. Additionally, understanding the different types of gasoline, such as regular, mid-grade, and premium, can help consumers make informed decisions about their fuel choices. By examining these aspects of gasoline production, we can gain a deeper understanding of how this essential fuel is created. Let's start by exploring the crude oil refining process, which is the foundation of gasoline production.
Crude Oil Refining Process
The crude oil refining process is a complex series of physical and chemical transformations that convert crude oil into various petroleum products, including gasoline, diesel fuel, jet fuel, and other fuels. The process begins with the receipt of crude oil at the refinery, where it is stored in tanks and prepared for processing. The first step in the refining process is separation, where the crude oil is heated and separated into different fractions, or types, of hydrocarbons based on their boiling points. This is typically done through a process known as fractional distillation, where the crude oil is heated and the different fractions are separated and collected. The resulting fractions include gasoline, naphtha, kerosene, diesel fuel, and residual fuel oil. The next step in the refining process is conversion, where the separated fractions are converted into more useful products through various chemical reactions. This can include processes such as cracking, reforming, and alkylation, which involve the use of catalysts and heat to break down the complex molecules in the crude oil into simpler, more useful compounds. The resulting products are then further refined and purified through various processes, including hydrotreating, isomerization, and blending. The final step in the refining process is product blending, where the various refined products are blended together to create the desired final products, such as gasoline, diesel fuel, and jet fuel. Throughout the refining process, various additives and chemicals are also added to the products to enhance their performance, stability, and safety. Overall, the crude oil refining process is a complex and highly technical process that requires careful planning, precise control, and a deep understanding of the underlying chemistry and physics.
Chemical Composition of Gasoline
Gasoline is a complex mixture of hydrocarbons, which are molecules composed of hydrogen and carbon atoms. The chemical composition of gasoline can vary depending on the source of the crude oil, the refining process, and the specific formulation of the gasoline. Typically, gasoline is a mixture of paraffins, naphthenes, aromatics, and olefins. Paraffins are saturated hydrocarbons with a single bond between carbon atoms, while naphthenes are saturated hydrocarbons with a ring structure. Aromatics are unsaturated hydrocarbons with a ring structure, and olefins are unsaturated hydrocarbons with a double bond between carbon atoms. The exact composition of gasoline can vary, but it is generally a mixture of around 50-60% paraffins, 20-30% naphthenes, 10-20% aromatics, and 5-10% olefins. Gasoline also contains additives such as detergents, dispersants, and anti-knock agents to improve its performance and stability. The chemical composition of gasoline is important because it affects the fuel's performance, efficiency, and emissions. For example, gasoline with a higher aromatic content can produce more emissions, while gasoline with a higher paraffin content can be more efficient. Understanding the chemical composition of gasoline is essential for optimizing its production, refining, and use in vehicles.
Types of Gasoline
Gasoline is a complex mixture of hydrocarbons, and its composition can vary depending on the refinery and the specific blend. There are several types of gasoline, each with its own unique characteristics and uses. Regular gasoline, also known as unleaded gasoline, is the most common type of gasoline and is suitable for most passenger vehicles. It typically has an octane rating of 87 and is a blend of various hydrocarbons, including paraffins, naphthenes, and aromatics. Mid-grade gasoline is a blend of regular and premium gasoline, with an octane rating of 89-90. It is designed for vehicles that require a higher octane fuel than regular gasoline but do not require premium gasoline. Premium gasoline, also known as high-octane gasoline, has an octane rating of 91-93 and is designed for high-performance vehicles that require a higher-octane fuel to run efficiently. It is typically more expensive than regular gasoline and is often used in luxury vehicles, sports cars, and other high-performance vehicles. Diesel gasoline is a type of fuel that is designed for diesel engines, which are commonly used in trucks, buses, and other heavy-duty vehicles. It has a higher energy density than regular gasoline and is typically more expensive. Alternative gasoline, such as ethanol and biodiesel, are becoming increasingly popular as a more environmentally friendly alternative to traditional gasoline. Ethanol is a biofuel that is made from fermented plant materials, such as corn and sugarcane, and can be blended with gasoline to create a more sustainable fuel. Biodiesel is a type of diesel fuel that is made from vegetable oils and animal fats, and can be used as a direct replacement for traditional diesel fuel. Overall, the type of gasoline used depends on the specific vehicle and its requirements, as well as personal preference and environmental concerns.
Extracting and Refining Crude Oil
The extraction and refining of crude oil is a complex process that involves several stages, from drilling and extraction to transportation and refining. The journey of crude oil from the ground to the refinery is a long and intricate one, requiring careful planning and execution. In this article, we will explore the various methods used to extract crude oil, the transportation methods employed to move it to refineries, and the refining process itself. We will begin by examining the drilling and extraction methods used to bring crude oil to the surface, including the different types of drilling rigs and extraction techniques employed. (Note: The supporting paragraph should be 200 words and the introduction should be 100 words)
Drilling and Extraction Methods
Drilling and extraction methods are crucial steps in the process of extracting crude oil from the earth. The most common method of drilling is rotary drilling, which involves using a rotating drill bit to create a hole in the ground. The drill bit is attached to a long pipe called a drill string, which is rotated by a motor at the surface. As the drill bit penetrates the earth, it creates a hole that is lined with steel casing to prevent collapse. Once the desired depth is reached, a perforating gun is used to create holes in the casing, allowing oil to flow into the well. Another method of drilling is directional drilling, which involves drilling at an angle to reach oil reservoirs that are not directly below the surface. This method is often used in offshore drilling, where the oil reservoirs are located beneath the seafloor. In addition to drilling, extraction methods such as primary recovery, secondary recovery, and tertiary recovery are used to extract oil from the well. Primary recovery involves allowing the oil to flow naturally from the well, while secondary recovery involves injecting water or gas into the well to push out more oil. Tertiary recovery, also known as enhanced oil recovery (EOR), involves using more advanced techniques such as injecting chemicals or heat into the well to extract even more oil. Overall, drilling and extraction methods play a critical role in the process of extracting crude oil from the earth, and advancements in these technologies have helped to increase oil production and reduce costs.
Transportation of Crude Oil
The transportation of crude oil is a critical step in the process of making gasoline. Once crude oil is extracted from the ground, it must be transported to a refinery where it can be processed into various petroleum products, including gasoline. There are several methods of transporting crude oil, including pipelines, tankers, and trucks. Pipelines are the most common method of transportation, as they are the safest and most efficient way to move large quantities of crude oil over long distances. Tankers are also widely used, particularly for transporting crude oil across oceans. Trucks are typically used for shorter distances and for transporting smaller quantities of crude oil. Regardless of the method of transportation, it is essential to ensure that the crude oil is handled and transported safely to prevent spills and other environmental hazards. This requires careful planning, specialized equipment, and trained personnel. In addition to safety concerns, the transportation of crude oil also has significant economic and environmental impacts. The cost of transportation can be a significant factor in the overall cost of producing gasoline, and the environmental impacts of transportation, such as air pollution and habitat disruption, must be carefully managed. Overall, the transportation of crude oil is a complex and critical step in the process of making gasoline, requiring careful planning, specialized equipment, and a commitment to safety and environmental responsibility.
Refining Process Overview
The refining process is a complex series of physical and chemical transformations that convert crude oil into a variety of petroleum products, including gasoline, diesel fuel, jet fuel, and lubricants. The process begins with the receipt of crude oil at the refinery, where it is stored in large tanks. The crude oil is then heated and separated into different fractions, or types of hydrocarbons, based on their boiling points. This process is called fractional distillation. The fractions are then further processed and transformed into various petroleum products through a series of chemical reactions, including cracking, reforming, and alkylation. Cracking involves breaking down large molecules into smaller ones, while reforming involves rearranging molecules to produce higher-octane gasoline. Alkylation combines smaller molecules to produce higher-octane gasoline and other petroleum products. The refining process also involves the removal of impurities, such as sulfur and nitrogen, through various treatment processes. The final products are then blended together to produce the desired grades of gasoline, diesel fuel, and other petroleum products. The refining process is a critical step in the production of gasoline and other petroleum products, and it requires careful control and monitoring to ensure that the products meet the required specifications and standards.
Converting Crude Oil into Gasoline
The conversion of crude oil into gasoline is a complex process that involves several stages. The journey from crude oil to gasoline is a fascinating one, and it's essential to understand the various processes involved. In this article, we will delve into the world of oil refining and explore the three primary stages of converting crude oil into gasoline: Catalytic Cracking Process, Hydrotreating and Reforming, and Blending and Additives. These stages are crucial in transforming crude oil into the gasoline that powers our vehicles. The first stage, Catalytic Cracking Process, is a critical step in breaking down the complex molecules of crude oil into simpler ones, making it possible to produce high-quality gasoline. This process involves the use of catalysts to crack the large molecules of crude oil into smaller ones, resulting in a higher yield of gasoline. Let's take a closer look at the Catalytic Cracking Process and how it plays a vital role in the production of gasoline.
Catalytic Cracking Process
The catalytic cracking process is a crucial step in converting crude oil into gasoline. This process involves the use of a catalyst, typically a zeolite or a metal oxide, to break down the complex molecules of crude oil into simpler, more valuable products such as gasoline, diesel fuel, and jet fuel. The process takes place in a fluidized bed reactor, where the crude oil is heated to high temperatures, typically between 400°C to 600°C, in the presence of the catalyst. The catalyst facilitates the cracking reaction, which involves the breaking of carbon-carbon bonds in the crude oil molecules, resulting in the formation of smaller molecules. The resulting products are then separated and processed further to produce high-quality gasoline and other petroleum products. The catalytic cracking process is a highly efficient and cost-effective method for converting crude oil into gasoline, and it is widely used in refineries around the world.
Hydrotreating and Reforming
Hydrotreating and reforming are two crucial processes in the petroleum refining industry that play a significant role in converting crude oil into gasoline. Hydrotreating is a process that involves the removal of impurities such as sulfur, nitrogen, and heavy metals from crude oil, while reforming is a process that converts low-octane naphtha into high-octane gasoline. In hydrotreating, crude oil is mixed with hydrogen and passed over a catalyst, which helps to remove impurities and improve the quality of the oil. The resulting product is a cleaner and more stable oil that can be further processed into various petroleum products, including gasoline. Reforming, on the other hand, involves the use of a catalyst to convert low-octane naphtha into high-octane gasoline. This process involves the rearrangement of the molecular structure of the naphtha, resulting in a higher-octane fuel that is more suitable for use in vehicles. The combination of hydrotreating and reforming processes enables refineries to produce high-quality gasoline that meets the required standards for use in vehicles. Overall, hydrotreating and reforming are essential processes in the production of gasoline, and their use has significantly improved the quality and performance of gasoline over the years.
Blending and Additives
Blending and additives are crucial steps in the process of converting crude oil into gasoline. Blending involves combining different types of gasoline to create a uniform product that meets specific standards and regulations. This process ensures that the final product has the desired octane rating, vapor pressure, and other characteristics. Additives, on the other hand, are chemicals that are added to the gasoline to enhance its performance, stability, and safety. These additives can include detergents, dispersants, and friction modifiers, which help to clean the engine, prevent corrosion, and improve fuel efficiency. The blending and additives process is carefully controlled to ensure that the final product meets the required specifications and is safe for use in vehicles. The use of additives also helps to reduce emissions and improve the overall environmental performance of the gasoline. By carefully selecting and blending the right additives, refiners can create a high-quality gasoline that meets the needs of drivers and the environment. Overall, blending and additives play a critical role in the production of gasoline, and their use is essential for creating a safe, efficient, and environmentally friendly fuel.