How Many Electrons Does Oxygen Have
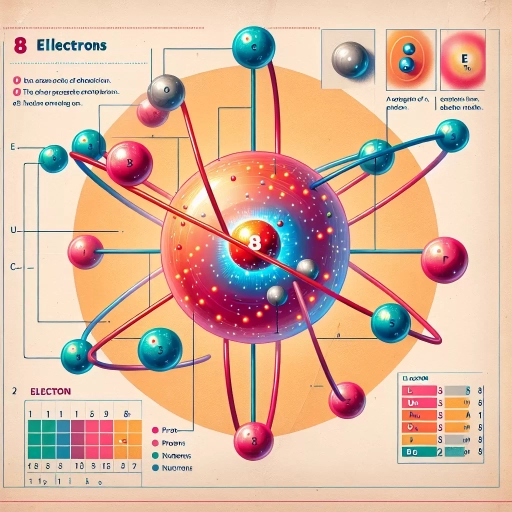
Oxygen, a vital element for life on Earth, plays a crucial role in countless chemical processes and biological functions. Understanding its atomic structure, particularly its electron count, is fundamental to comprehending its behavior and significance in various scientific fields. This article delves into the question: "How many electrons does oxygen have?" We'll explore this topic in depth, starting with an examination of oxygen's atomic structure, which forms the foundation for understanding its electron configuration. Next, we'll investigate the specific electron arrangement of oxygen, focusing on its valence electrons and their importance in chemical interactions. Finally, we'll discuss the far-reaching implications of oxygen's electron count in both chemistry and biology, highlighting how this seemingly simple aspect of atomic structure influences complex phenomena in the natural world. By unraveling the mysteries of oxygen's electrons, we can gain valuable insights into the element's reactivity, bonding capabilities, and essential role in sustaining life. Let's begin our journey by first understanding the atomic structure of oxygen, which will provide the necessary context for our exploration of its electron count.
Understanding the Atomic Structure of Oxygen
Oxygen, the life-sustaining element that surrounds us, plays a crucial role in our everyday existence. Yet, beyond its vital importance to living organisms, oxygen possesses a fascinating atomic structure that reveals the intricate nature of matter itself. To truly appreciate the complexity of this essential element, we must delve into the world of subatomic particles and explore the fundamental building blocks that make up an oxygen atom. This journey begins with understanding the basic components of an atom: protons, neutrons, and electrons, which form the foundation of all elements. From there, we'll examine oxygen's unique atomic number and its significance in determining the element's properties and behavior. Finally, we'll unravel the arrangement of electrons in oxygen's electron shells, shedding light on its chemical reactivity and bonding capabilities. By exploring these key aspects, we can gain a deeper appreciation for the atomic structure of oxygen and how it influences its role in our world. Let us embark on this atomic adventure to uncover the secrets hidden within the microscopic realm of Understanding the Atomic Structure of Oxygen.
The basic components of an atom: protons, neutrons, and electrons
At the heart of every atom, including oxygen, lies a fascinating world of subatomic particles. The basic components of an atom are protons, neutrons, and electrons, each playing a crucial role in determining the atom's properties and behavior. To truly understand the atomic structure of oxygen, it's essential to grasp the nature and function of these fundamental particles.
Protons are positively charged particles found in the nucleus of an atom. They contribute significantly to the atom's mass and are responsible for its atomic number, which defines the element's identity. In the case of oxygen, there are always 8 protons in its nucleus, giving it its unique chemical properties. Neutrons, on the other hand, are electrically neutral particles also located in the nucleus. They work alongside protons to provide stability to the atomic nucleus and contribute to the atom's mass. The number of neutrons can vary in different isotopes of oxygen, but the most common form has 8 neutrons.
Electrons are the third key component of an atom, and they are particularly relevant when discussing how many electrons oxygen has. These negatively charged particles orbit the nucleus in shells or energy levels. Electrons are much smaller and lighter than protons and neutrons, but they play a crucial role in chemical bonding and determining an element's reactivity. In a neutral atom, the number of electrons equals the number of protons, maintaining overall electrical neutrality. For oxygen, this means there are typically 8 electrons surrounding the nucleus.
The arrangement of these electrons in shells is vital to understanding oxygen's behavior in chemical reactions. Electrons occupy different energy levels, with the first shell holding up to 2 electrons, and the second shell accommodating up to 8. In oxygen's case, 2 electrons fill the first shell, and 6 occupy the second shell. This configuration leaves oxygen two electrons short of a full outer shell, making it highly reactive and eager to form bonds with other elements to achieve a stable octet.
Understanding the interplay between protons, neutrons, and electrons is crucial for grasping concepts like ionic and covalent bonding, oxidation states, and molecular geometry. For instance, oxygen's tendency to gain two electrons to complete its outer shell explains its role in forming compounds like water (H2O) and its importance in biological processes such as respiration. The balance and arrangement of these subatomic particles not only define oxygen's place in the periodic table but also govern its behavior in countless chemical reactions that are fundamental to life and the physical world around us.
Oxygen's atomic number and its significance
Oxygen's atomic number is a fundamental characteristic that plays a crucial role in understanding its atomic structure and chemical behavior. With an atomic number of 8, oxygen occupies a unique position in the periodic table, offering insights into its electron configuration and bonding properties. This atomic number signifies that oxygen atoms possess 8 protons in their nucleus, a feature that distinguishes oxygen from all other elements and determines many of its physical and chemical properties. The significance of oxygen's atomic number extends far beyond mere identification. It directly influences the element's electron arrangement, which is key to its reactivity and role in countless chemical processes. With 8 protons in its nucleus, a neutral oxygen atom must also have 8 electrons to maintain electrical neutrality. These electrons are distributed across energy levels, or shells, following the principles of quantum mechanics. The first two electrons occupy the innermost shell, while the remaining six reside in the second shell. This electron configuration, 1s² 2s² 2p⁴, is responsible for oxygen's high electronegativity and its tendency to form covalent bonds with other elements. The atomic number also explains oxygen's position in the periodic table as the first element in Group 16, also known as the chalcogens. This placement reflects its valence electron count of six, which drives its chemical behavior. Oxygen's strong tendency to acquire two additional electrons to complete its outer shell leads to its role as a powerful oxidizing agent and its ability to form compounds with most other elements. This property is fundamental to many biological processes, including cellular respiration, where oxygen serves as the final electron acceptor in the electron transport chain. Furthermore, the atomic number of oxygen is intrinsically linked to its isotopic variations. While the most common isotope of oxygen has 8 neutrons (matching its 8 protons), other stable isotopes exist with different neutron counts. These isotopes, particularly oxygen-18, are valuable tools in scientific research, including climate studies and medical diagnostics. The ratio of oxygen isotopes in ice cores, for example, provides crucial data on historical climate patterns. Understanding oxygen's atomic number is also essential in fields such as astrophysics and cosmology. As the third most abundant element in the universe by mass, oxygen's formation through stellar nucleosynthesis is a key topic in the study of stellar evolution and the chemical composition of galaxies. The production of oxygen in stars requires specific conditions related to its atomic structure, highlighting the far-reaching implications of its atomic number. In conclusion, the atomic number of oxygen is not just a simple identifier but a gateway to understanding its complex role in chemistry, biology, and the universe at large. From its electron configuration to its isotopic variations, every aspect of oxygen's behavior can be traced back to this fundamental characteristic, underscoring its significance in scientific study and our understanding of the natural world.
The arrangement of electrons in oxygen's electron shells
The arrangement of electrons in oxygen's electron shells is a fascinating aspect of its atomic structure, providing crucial insights into its chemical behavior and bonding capabilities. Oxygen, with its atomic number of 8, possesses a total of 8 electrons that are distributed across two electron shells. This arrangement follows the principles of electron configuration and the Aufbau principle, which dictate how electrons fill orbitals in an atom. The first electron shell, also known as the K shell, is the innermost shell and can hold a maximum of two electrons. In oxygen, this shell is completely filled with two electrons. These electrons are tightly bound to the nucleus and are not typically involved in chemical reactions. The remaining six electrons occupy the second electron shell, also called the L shell. This shell can accommodate up to eight electrons, but in oxygen's case, it is only partially filled. The second shell of oxygen contains four orbitals: one 2s orbital and three 2p orbitals. The 2s orbital is spherical and is filled first with two electrons. The remaining four electrons occupy the three 2p orbitals, which have a dumbbell-like shape. These p orbitals are oriented along the x, y, and z axes, providing oxygen with its characteristic three-dimensional electron distribution. This specific arrangement of electrons in oxygen's outer shell is crucial to understanding its chemical properties. With six electrons in its outermost shell, oxygen is two electrons short of achieving a stable octet configuration. This electron deficiency makes oxygen highly reactive, as it tends to either gain or share electrons to complete its octet. This property explains oxygen's ability to form covalent bonds with other elements and its role as a powerful oxidizing agent. The electron configuration of oxygen can be written as 1s² 2s² 2p⁴, where the superscripts indicate the number of electrons in each orbital. This notation provides a concise representation of how electrons are distributed across the shells and subshells. Understanding this configuration is essential for predicting oxygen's behavior in chemical reactions and its ability to form various compounds. The arrangement of electrons also influences oxygen's magnetic properties. In its ground state, oxygen has two unpaired electrons in its 2p orbitals, giving it a paramagnetic nature. This property is responsible for oxygen's behavior in magnetic fields and its crucial role in many biological processes, including its ability to bind to hemoglobin in blood. In conclusion, the arrangement of electrons in oxygen's shells is a prime example of how atomic structure determines chemical and physical properties. This knowledge is fundamental to understanding oxygen's reactivity, bonding behavior, and its vital role in countless chemical and biological processes that sustain life on Earth.
Electron Configuration and Valence Electrons of Oxygen
Oxygen, the life-sustaining element that makes up approximately 21% of Earth's atmosphere, plays a crucial role in countless biological and chemical processes. To truly appreciate its significance, one must delve into the atomic structure of oxygen, particularly its electron configuration and valence electrons. This article explores the fascinating world of oxygen's atomic makeup, shedding light on the intricate arrangement of its electrons and how this configuration influences its chemical behavior. We will begin by examining the fundamental concepts of electron configuration and energy levels, providing a solid foundation for understanding atomic structure. Then, we'll focus on oxygen's specific electron configuration, detailing the precise arrangement of its electrons across different orbitals. Furthermore, we'll investigate the importance of valence electrons in chemical bonding, explaining how these outermost electrons determine oxygen's reactivity and its ability to form compounds. By unraveling these atomic intricacies, we can gain a deeper appreciation for the element that sustains life on our planet. Join us as we embark on a journey to understand the atomic structure of oxygen, uncovering the secrets hidden within its electrons.
The concept of electron configuration and energy levels
The concept of electron configuration and energy levels is fundamental to understanding the behavior of electrons within atoms, particularly in the case of oxygen. This knowledge is crucial for comprehending chemical bonding, reactivity, and various atomic properties. Electrons in an atom are arranged in distinct energy levels, also known as electron shells or principal quantum numbers. These levels are typically labeled with numbers (1, 2, 3, etc.) or letters (K, L, M, etc.). As we move away from the nucleus, each successive energy level has higher energy and can accommodate more electrons. The distribution of electrons among these energy levels follows specific rules and patterns, which collectively form the atom's electron configuration. The Aufbau principle, Pauli exclusion principle, and Hund's rule govern how electrons fill these energy levels. According to the Aufbau principle, electrons occupy the lowest available energy levels first before moving to higher levels. The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers, effectively limiting each orbital to a maximum of two electrons with opposite spins. Hund's rule dictates that electrons in the same sublevel (s, p, d, or f) will occupy separate orbitals with parallel spins before pairing up. In the case of oxygen, with its atomic number of 8, the electron configuration is 1s² 2s² 2p⁴. This notation describes how the eight electrons are distributed: two in the first energy level (1s orbital), two in the second energy level's s orbital (2s), and four in the second energy level's p orbitals (2p). The superscript numbers indicate the number of electrons in each orbital. Understanding electron configuration is particularly important when examining an atom's valence electrons – those in the outermost shell responsible for chemical bonding. For oxygen, the six electrons in the second energy level (2s² 2p⁴) are its valence electrons, explaining its tendency to form covalent bonds and its role in numerous chemical reactions. The concept of energy levels and electron configuration also helps explain periodic trends in atomic properties. As we move across a period in the periodic table, the number of electrons in the outermost shell increases, affecting properties such as atomic radius, ionization energy, and electronegativity. Similarly, moving down a group, the number of electron shells increases, influencing an element's reactivity and bonding behavior. In summary, the electron configuration and energy levels provide a comprehensive framework for understanding atomic structure and behavior. This knowledge is indispensable for predicting and explaining chemical properties, bonding patterns, and reactivity of elements like oxygen, making it a cornerstone concept in chemistry and related scientific fields.
Oxygen's specific electron configuration
Oxygen's specific electron configuration is a fascinating aspect of its atomic structure that plays a crucial role in its chemical behavior and reactivity. As the eighth element in the periodic table, oxygen has a total of eight electrons orbiting its nucleus. These electrons are arranged in specific energy levels and orbitals, following the principles of quantum mechanics and the Aufbau principle. The electron configuration of oxygen can be written as 1s² 2s² 2p⁴. This notation provides a detailed map of how the electrons are distributed across the atom's orbitals. Breaking it down, we see that oxygen has two electrons in its innermost 1s orbital, which is closest to the nucleus and has the lowest energy. The next energy level contains the 2s orbital, which is filled with two electrons, and the 2p orbitals, which contain four electrons. The 2p orbitals are particularly interesting in oxygen's configuration. There are three 2p orbitals (2px, 2py, and 2pz), each capable of holding two electrons. In oxygen's ground state, these four electrons are distributed across the three 2p orbitals following Hund's rule, which states that electrons will occupy orbitals of equal energy individually before pairing up. This results in two of the 2p orbitals containing one electron each, and the third 2p orbital containing two paired electrons. This specific arrangement of electrons gives oxygen its unique chemical properties. The six electrons in the outermost shell (2s² 2p⁴) are considered valence electrons, which are responsible for forming chemical bonds. Oxygen's tendency to gain two electrons to achieve a stable octet configuration makes it highly electronegative, allowing it to form strong covalent bonds with many other elements. Understanding oxygen's electron configuration is essential in predicting its behavior in chemical reactions, its role in biological processes, and its importance in various industrial applications. For instance, the presence of unpaired electrons in its 2p orbitals makes oxygen paramagnetic, a property that is utilized in magnetic resonance imaging (MRI) technology. Additionally, the electron configuration explains oxygen's ability to form double bonds, as seen in the O₂ molecule, which is crucial for life on Earth. In summary, oxygen's specific electron configuration of 1s² 2s² 2p⁴ provides deep insights into its atomic structure and chemical behavior. This arrangement of electrons, particularly in the valence shell, is fundamental to understanding oxygen's reactivity, bonding capabilities, and its pivotal role in countless chemical and biological processes that shape our world.
The importance of valence electrons in chemical bonding
Valence electrons play a pivotal role in chemical bonding, serving as the primary actors in the formation of chemical compounds and determining the reactivity of elements. These outermost electrons, located in the highest occupied energy level of an atom, are the most accessible for interaction with other atoms. In the case of oxygen, which has six valence electrons, understanding their behavior is crucial to comprehending its chemical properties and bonding tendencies. The importance of valence electrons in chemical bonding cannot be overstated. They determine an element's ability to form chemical bonds, influencing its reactivity and the types of compounds it can create. For oxygen, its six valence electrons make it highly reactive, as it seeks to achieve a stable octet configuration by gaining or sharing electrons. This electron configuration drives oxygen's tendency to form covalent bonds with other elements, particularly in molecules like water (H2O) and carbon dioxide (CO2), which are essential for life on Earth. Valence electrons also dictate the oxidation states an element can assume. Oxygen, with its six valence electrons, commonly exhibits a -2 oxidation state in many compounds, as it tends to gain two electrons to complete its octet. This property is fundamental in understanding oxygen's role in oxidation-reduction reactions, which are ubiquitous in biological processes and industrial applications. Furthermore, the number and arrangement of valence electrons influence an element's electronegativity, which is the ability to attract electrons in a chemical bond. Oxygen's high electronegativity, due to its valence electron configuration, explains its strong tendency to form polar covalent bonds and its significance in hydrogen bonding. These characteristics are crucial in determining the properties of water, including its high boiling point and surface tension, which are vital for life processes. The concept of valence electrons also helps explain periodic trends such as atomic size, ionization energy, and electron affinity. As we move across the periodic table, the increasing number of valence electrons generally correlates with higher electronegativity and reactivity, until a noble gas configuration is reached. This understanding is essential for predicting chemical behavior and designing new materials with specific properties. In molecular orbital theory, valence electrons participate in forming bonding and antibonding orbitals, which determine the stability and electronic properties of molecules. For oxygen, the distribution of electrons in these molecular orbitals explains phenomena such as the paramagnetic nature of molecular oxygen (O2) and its ability to act as an oxidizing agent. By comprehending the role of valence electrons in oxygen and other elements, scientists can predict and manipulate chemical reactions, design more efficient catalysts, and develop new materials with tailored properties. This knowledge is fundamental in fields ranging from materials science and nanotechnology to biochemistry and environmental science, underscoring the far-reaching importance of valence electrons in our understanding of the chemical world.
Implications of Oxygen's Electron Count in Chemistry and Biology
Oxygen, a fundamental element essential for life on Earth, plays a pivotal role in countless chemical and biological processes. Its unique electron configuration, with six valence electrons, profoundly influences its behavior and interactions with other elements. This article delves into the implications of oxygen's electron count in both chemistry and biology, exploring how this atomic characteristic shapes the world around us. We will examine oxygen's role in forming compounds and molecules, highlighting its ability to form covalent bonds and participate in various chemical reactions. Additionally, we'll investigate the significance of oxygen's electron count in biological processes, including its crucial function in cellular respiration and energy production. Furthermore, we'll analyze how oxygen's electron configuration influences its reactivity and properties, shedding light on its importance in oxidation reactions and its role as an electron acceptor. By understanding these key aspects, we can gain a deeper appreciation for the intricate ways in which oxygen's electron count impacts both the chemical and biological realms. To fully grasp these concepts, it is essential to first understand the atomic structure of oxygen, which serves as the foundation for its unique properties and behaviors.
Oxygen's role in forming compounds and molecules
Oxygen's unique electronic configuration, with six valence electrons, plays a pivotal role in its ability to form a wide variety of compounds and molecules essential to life and numerous chemical processes. This characteristic makes oxygen one of the most versatile and important elements in chemistry and biology. The element's high electronegativity and tendency to form covalent bonds allow it to participate in numerous chemical reactions, creating both simple and complex molecules that are fundamental to our world. In organic chemistry, oxygen is a key player in forming functional groups such as alcohols, ethers, aldehydes, ketones, carboxylic acids, and esters. These groups are essential in the structure and function of many biological molecules, including carbohydrates, lipids, and proteins. For example, the hydroxyl group (-OH) found in alcohols is crucial for the solubility and reactivity of many organic compounds. In carbohydrates, oxygen atoms form glycosidic bonds between sugar molecules, creating complex structures like cellulose and starch, which are vital for energy storage and structural support in plants. Oxygen's role in inorganic chemistry is equally significant. It forms oxides with most elements, contributing to the formation of minerals in the Earth's crust and atmosphere. Metal oxides, such as iron oxide (rust) and aluminum oxide, are ubiquitous in nature and have numerous industrial applications. In the atmosphere, oxygen molecules (O₂) are essential for respiration in many organisms, while ozone (O₃) in the stratosphere protects life on Earth from harmful ultraviolet radiation. The element's ability to form double bonds is crucial in many biological processes. In DNA and RNA, oxygen atoms participate in the phosphodiester bonds that link nucleotides, forming the backbone of these genetic molecules. Additionally, oxygen is a key component of phospholipids, which form cell membranes, allowing for the compartmentalization of cellular processes. Oxygen's role in forming hydrogen bonds is another critical aspect of its chemical behavior. These weak but numerous interactions are essential for the structure and function of water, proteins, and nucleic acids. In water, hydrogen bonding gives rise to its unique properties, such as high boiling point and surface tension, which are crucial for life processes. In biochemistry, oxygen's ability to accept electrons makes it a terminal electron acceptor in cellular respiration, a process fundamental to energy production in aerobic organisms. This property also makes oxygen a powerful oxidizing agent, participating in countless redox reactions in living systems and industrial processes. Understanding oxygen's role in forming compounds and molecules is crucial for fields ranging from materials science to pharmaceutical development. Its versatility in bonding and its abundance in the Earth's crust and atmosphere make it an indispensable element in shaping the world as we know it, underscoring the profound implications of its electron count in chemistry and biology.
The significance of oxygen's electron count in biological processes
Oxygen's electron count plays a crucial role in numerous biological processes, making it an indispensable element for life as we know it. With six valence electrons, oxygen has a unique ability to form covalent bonds and participate in various chemical reactions that are essential for living organisms. This electronic configuration is the key to understanding oxygen's significance in biological systems and its impact on the fundamental processes that sustain life. One of the most important implications of oxygen's electron count is its role in cellular respiration. The process of aerobic respiration, which is the primary means by which most organisms generate energy, relies heavily on oxygen's ability to accept electrons. As the final electron acceptor in the electron transport chain, oxygen's six valence electrons allow it to readily accept four additional electrons, forming water molecules. This electron transfer process is crucial for the production of adenosine triphosphate (ATP), the energy currency of cells. Without oxygen's unique electronic properties, this efficient energy production mechanism would not be possible, severely limiting the metabolic capabilities of complex organisms. Furthermore, oxygen's electron count contributes to its role in the formation of reactive oxygen species (ROS). While ROS can be harmful in excess, they also serve important functions in cell signaling and immune responses. The ability of oxygen to partially accept electrons or undergo reduction reactions is directly related to its electronic configuration, allowing for the formation of superoxide, hydrogen peroxide, and hydroxyl radicals. These molecules play critical roles in cellular processes such as apoptosis, gene expression regulation, and defense against pathogens. Oxygen's electron count also influences its behavior in biological molecules, particularly in proteins. Many enzymes rely on oxygen or oxygen-containing molecules as cofactors or substrates. The electronic properties of oxygen allow it to form coordinate covalent bonds with metal ions, such as iron in hemoglobin or copper in cytochrome c oxidase. These interactions are essential for the transport and utilization of oxygen throughout the body and for maintaining the proper function of numerous metabolic pathways. In addition, the electron configuration of oxygen is fundamental to the structure and function of biomolecules. Oxygen's ability to form hydrogen bonds, due to its electronegativity and lone pair electrons, is crucial for maintaining the three-dimensional structure of proteins, nucleic acids, and other macromolecules. These hydrogen bonds contribute to the stability of DNA double helices, protein folding, and the properties of water as a biological solvent. The significance of oxygen's electron count extends to photosynthesis as well. In this process, which is the basis for most life on Earth, oxygen is produced as a byproduct of water splitting. The electronic properties of oxygen allow it to be released as a stable diatomic molecule, while the electrons from water are used to reduce carbon dioxide into organic compounds. This cycle of oxygen production and consumption, driven by the element's electronic characteristics, has shaped the Earth's atmosphere and the evolution of life over billions of years.
How oxygen's electron configuration influences its reactivity and properties
Oxygen's electron configuration plays a crucial role in determining its reactivity and properties, making it one of the most important elements in chemistry and biology. With six valence electrons in its outer shell, oxygen exhibits a unique balance between stability and reactivity that influences its behavior in various chemical and biological processes. The electron configuration of oxygen (1s² 2s² 2p⁴) leaves it two electrons short of a full octet, which drives its tendency to form covalent bonds with other atoms. This electronic arrangement makes oxygen highly electronegative, meaning it has a strong ability to attract electrons from other elements. As a result, oxygen readily forms compounds with a wide range of elements, particularly metals and nonmetals, leading to the creation of oxides, hydroxides, and numerous organic compounds essential for life. The reactivity of oxygen is further enhanced by its ability to exist in different allotropic forms, such as diatomic oxygen (O₂) and ozone (O₃). In its most common form, O₂, oxygen molecules share a double bond, which contributes to its stability while still allowing for chemical reactions. This balance between stability and reactivity is crucial for oxygen's role in respiration, where it can be transported through the bloodstream and utilized in cellular processes without spontaneously combusting. Oxygen's electron configuration also influences its magnetic properties. The presence of two unpaired electrons in its outermost orbital makes oxygen paramagnetic, allowing it to be slightly attracted to magnetic fields. This property has important implications in medical imaging techniques such as MRI (Magnetic Resonance Imaging), where the magnetic behavior of oxygen in blood can be used to detect and visualize physiological processes. The electron arrangement of oxygen also explains its ability to form hydrogen bonds, which are essential for the unique properties of water. The lone pairs of electrons on oxygen atoms in water molecules can interact with hydrogen atoms from neighboring molecules, creating a network of weak but significant interactions. These hydrogen bonds are responsible for water's high boiling point, surface tension, and ability to dissolve a wide range of substances, all of which are crucial for biological processes. In organic chemistry, oxygen's electron configuration allows it to participate in various types of bonding and functional groups. It can form single, double, or even triple bonds with carbon atoms, leading to a diverse array of organic compounds such as alcohols, ethers, aldehydes, ketones, and carboxylic acids. Each of these functional groups exhibits distinct properties and reactivities, contributing to the vast complexity of organic molecules and their functions in living organisms. Understanding oxygen's electron configuration and its implications is fundamental to fields such as materials science, environmental chemistry, and biochemistry. It helps explain phenomena ranging from metal corrosion and combustion reactions to the intricate processes of photosynthesis and cellular respiration. By grasping the fundamental principles of oxygen's electronic structure, scientists can better predict and manipulate chemical reactions, design new materials, and develop innovative technologies that harness the unique properties of this essential element.