How Many Protons Does Sodium Have
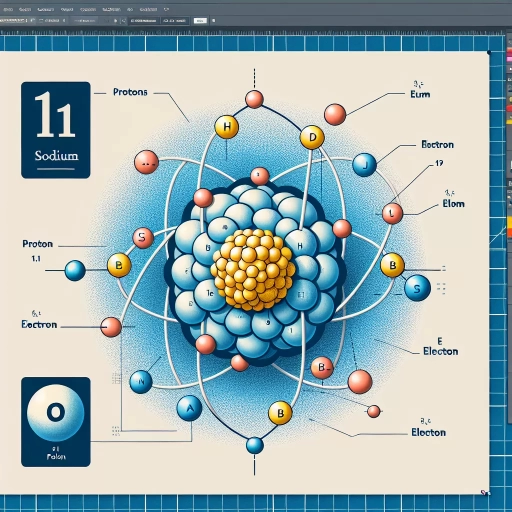
Sodium is a chemical element that is widely recognized for its role in various biological and chemical processes. It is a key component in the manufacture of many products, including paper, dyes, and soaps. But have you ever wondered what makes sodium tick at the atomic level? To answer this question, we need to delve into the atomic structure of sodium and explore its composition. This involves understanding the number of protons that sodium has, which is a crucial aspect of its chemical properties. In this article, we will explore the atomic structure of sodium, identify the number of protons it has, and discuss the implications of its proton count in chemistry. By understanding these concepts, we can gain a deeper appreciation for the importance of sodium in various fields. So, let's start by understanding the atomic structure of sodium.
Understanding the Atomic Structure of Sodium
Sodium, a chemical element with the symbol Na, is a soft, silvery-white, highly reactive metal. Understanding the atomic structure of sodium is crucial in comprehending its chemical properties and behavior. The atomic structure of sodium is composed of protons, neutrons, and electrons, which play a significant role in determining its chemical properties. The atomic number of sodium, which is 11, is a key factor in understanding its atomic structure. In this article, we will delve into the atomic structure of sodium, exploring its atomic number and its significance, the composition of protons, neutrons, and electrons in an atom, and how the atomic structure relates to its chemical properties. By understanding these concepts, we can gain a deeper insight into the behavior of sodium and its applications in various fields. The atomic number of sodium, being a fundamental aspect of its atomic structure, will be our starting point in this exploration.
Atomic Number and Its Significance
The atomic number of an element is a unique identifier that defines its position in the periodic table and determines its chemical properties. It is the number of protons present in the nucleus of an atom, which in turn determines the number of electrons in a neutral atom. The atomic number is a fundamental concept in chemistry and physics, and it plays a crucial role in understanding the structure and behavior of atoms. In the case of sodium, its atomic number is 11, which means that it has 11 protons in its nucleus. This number of protons determines the chemical properties of sodium, such as its reactivity and the types of compounds it can form. Understanding the atomic number of sodium is essential in understanding its atomic structure and behavior, and it has numerous applications in various fields, including chemistry, physics, and materials science.
Protons, Neutrons, and Electrons in an Atom
Protons, neutrons, and electrons are the three main subatomic particles that make up an atom. Protons and neutrons are found in the nucleus, which is the central part of the atom, while electrons orbit around the nucleus. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms. For example, sodium has 11 protons in its atoms. The number of neutrons in an atom can vary, leading to different isotopes of the same element. Electrons are arranged in energy levels or shells around the nucleus, and the number of electrons in an atom is equal to the number of protons. In a neutral atom, the number of protons and electrons is equal, and the positive and negative charges balance each other out. Understanding the structure of protons, neutrons, and electrons in an atom is crucial for understanding the properties and behavior of elements, including sodium.
How Atomic Structure Relates to Chemical Properties
The atomic structure of an element plays a crucial role in determining its chemical properties. The arrangement of electrons in an atom, particularly the outermost energy level, is responsible for an element's reactivity and its ability to form bonds with other elements. In the case of sodium, its atomic structure consists of 11 protons, 12 neutrons, and 11 electrons, with one electron in the outermost energy level. This single outer electron makes sodium highly reactive, as it readily loses this electron to form a positive ion, known as a cation. This reactivity is a key factor in sodium's ability to form compounds with other elements, such as chlorine, to produce table salt. The atomic structure of sodium also influences its physical properties, such as its melting and boiling points, which are relatively low due to the weak electrostatic forces between sodium ions. Understanding the atomic structure of sodium is essential in explaining its chemical properties and behavior, and is a fundamental concept in chemistry.
Identifying the Number of Protons in Sodium
Sodium, a chemical element with the symbol Na, is a soft, silvery-white, highly reactive metal. Identifying the number of protons in sodium is crucial in understanding its chemical properties and behavior. To determine the number of protons in sodium, we can rely on three key concepts: the role of the periodic table in identifying protons, understanding isotopes and their proton count, and calculating protons from atomic mass and neutrons. By exploring these concepts, we can gain a deeper understanding of sodium's atomic structure. First, we will examine how the periodic table serves as a valuable resource in identifying the number of protons in sodium, providing a straightforward and efficient method for determining its atomic number.
The Role of the Periodic Table in Identifying Protons
The periodic table is a powerful tool in identifying the number of protons in an atom, including sodium. The periodic table is arranged in a way that elements with the same number of protons in their atomic nuclei are placed in the same group, or column. The elements in each group have the same number of electrons in their outermost energy level, which determines their chemical properties. By looking at the periodic table, we can identify the group number of an element, which corresponds to the number of protons in its atomic nucleus. In the case of sodium, it is located in group 1 of the periodic table, which means it has one proton in its atomic nucleus. However, this is not entirely accurate, as sodium actually has 11 protons in its atomic nucleus. The reason for this discrepancy is that the periodic table is arranged based on the number of electrons in an element's outermost energy level, not the number of protons in its atomic nucleus. Nevertheless, the periodic table is still a useful tool in identifying the number of protons in an atom, as it provides a general idea of the number of protons in an element's atomic nucleus. By combining the information from the periodic table with other knowledge, such as the atomic number of an element, we can accurately determine the number of protons in an atom.
Understanding Isotopes and Their Proton Count
Isotopes are atoms of the same element that have the same number of protons in their atomic nuclei but differ in the number of neutrons. This variation in neutron count affects the overall mass of the atom, resulting in different isotopes of the same element. The number of protons in an atom's nucleus, also known as the atomic number, defines the element's identity in the periodic table. For instance, sodium has an atomic number of 11, indicating that it has 11 protons in its atomic nucleus. However, sodium has several isotopes, including sodium-22, sodium-23, and sodium-24, which have 11 protons but varying numbers of neutrons. Understanding isotopes and their proton count is crucial in chemistry and physics, as it helps scientists identify and distinguish between different elements and their isotopes. By recognizing the unique proton count of an element, researchers can determine its position in the periodic table and predict its chemical properties. In the case of sodium, its 11 protons determine its reactivity and behavior in chemical reactions. Therefore, identifying the number of protons in sodium is essential for understanding its chemical properties and behavior.
Calculating Protons from Atomic Mass and Neutrons
The atomic mass of an element is the total number of protons and neutrons present in its atomic nucleus. To calculate the number of protons in an atom, we need to know the atomic mass and the number of neutrons. The atomic mass of sodium is 22.9898 u (unified atomic mass units), and the number of neutrons in a sodium atom is 12. To calculate the number of protons, we can use the formula: number of protons = atomic mass - number of neutrons. Plugging in the values, we get: number of protons = 22.9898 - 12 = 10.9898. Since the number of protons must be a whole number, we round down to the nearest whole number, which gives us 11 protons. Therefore, a sodium atom has 11 protons.
Implications of Sodium's Proton Count in Chemistry
The number of protons in an atom's nucleus, also known as the atomic number, plays a crucial role in determining the chemical properties of an element. In the case of sodium, its proton count has significant implications for its chemical reactivity, electron configuration, and position in the periodic table. The number of protons in sodium's nucleus affects its ability to form bonds with other elements, which in turn influences its chemical reactivity. Furthermore, the proton count of sodium also determines its electron configuration, which is essential for understanding its chemical behavior. Additionally, the position of sodium in the periodic table is directly related to its proton count, which provides valuable information about its chemical properties. Understanding the implications of sodium's proton count is essential for chemists and researchers, as it allows them to predict and explain the chemical behavior of this element. This article will explore the implications of sodium's proton count in chemistry, starting with its impact on chemical reactivity and the number of protons.
Chemical Reactivity and the Number of Protons
Chemical reactivity is deeply connected to the number of protons in an atom's nucleus, as it determines the element's position in the periodic table and its electron configuration. The number of protons, also known as the atomic number, defines the identity of an element and its chemical properties. In the case of sodium, its 11 protons dictate its reactivity, particularly its tendency to lose one electron to form a positive ion, or cation. This characteristic makes sodium highly reactive, especially with water, as it readily donates an electron to form sodium hydroxide and hydrogen gas. The number of protons in sodium also influences its ability to form compounds with other elements, such as chlorine, to create table salt. Understanding the relationship between an element's proton count and its chemical reactivity is crucial in predicting and explaining the behavior of elements in various chemical reactions.
Electron Configuration and Its Relation to Protons
Electron configuration is a crucial concept in chemistry that describes the arrangement of electrons in an atom. It is closely related to the number of protons in an atom, as the number of protons determines the number of electrons in a neutral atom. In the case of sodium, which has 11 protons, the electron configuration is 1s² 2s² 2p⁶ 3s¹. This configuration indicates that the outermost energy level of sodium has one electron, which is responsible for its chemical reactivity. The electron configuration of an atom is essential in understanding its chemical properties and behavior, as it determines the number of electrons available for bonding and the types of bonds that can be formed. In the context of sodium, its electron configuration plays a significant role in its reactivity, particularly in its ability to readily lose one electron to form a positive ion. This property makes sodium highly reactive and useful in various chemical reactions.
How Proton Count Affects Sodium's Position in the Periodic Table
Sodium's proton count of 11 significantly influences its position in the periodic table. As a member of the alkali metal group, sodium is situated in the first column of the periodic table, alongside other elements with a single electron in their outermost energy level. The proton count of 11 determines sodium's atomic number, which in turn dictates its placement in the periodic table. With 11 protons, sodium has a relatively low atomic number, resulting in its position in the third period of the periodic table. This placement is crucial, as it reflects sodium's chemical properties and behavior. The proton count of 11 also affects sodium's electron configuration, which is characterized by a single electron in the outermost energy level. This electron configuration is responsible for sodium's high reactivity, as it readily loses one electron to form a positive ion. The unique combination of sodium's proton count and electron configuration makes it an essential element in various chemical reactions and applications.