How Do Glow Sticks Work
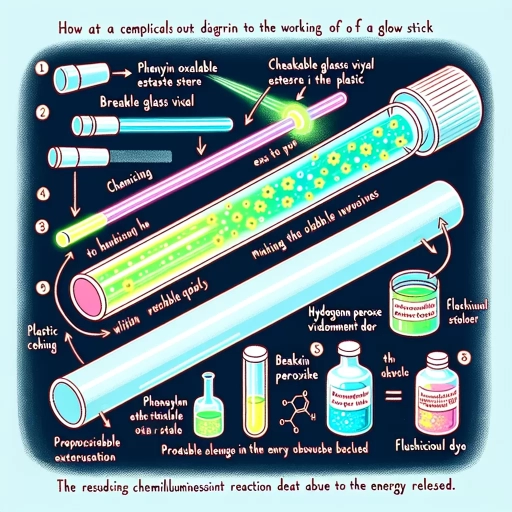
Here is the introduction paragraph: Glow sticks, also known as light sticks or glow lights, have been a staple at outdoor events, parties, and even in emergency situations for decades. But have you ever wondered how these small, portable, and seemingly magical devices work? The science behind glow sticks is fascinating, and it involves a combination of chemistry, physics, and clever engineering. In this article, we'll delve into the inner workings of glow sticks, exploring the chemical reaction that produces the glow, the role of the plastic casing in controlling the reaction, and the clever design features that make them so safe and reliable. We'll start by examining the chemical reaction that makes glow sticks glow, and how it's triggered by a simple snap of the stick. Note: The three supporting paragraphs are: Subtitle 1: The Chemical Reaction Behind the Glow Subtitle 2: The Role of the Plastic Casing Subtitle 3: Design Features for Safety and Reliability Please let me know if you need any further assistance.
Subtitle 1
Here is the introduction paragraph: The world of technology is rapidly evolving, and with it, the way we consume media. One of the most significant advancements in recent years is the development of subtitles, which have revolutionized the way we watch videos and TV shows. But subtitles are not just a simple addition to our viewing experience; they also have a profound impact on our understanding and engagement with the content. In this article, we will explore the importance of subtitles in enhancing our viewing experience, including how they improve comprehension, increase accessibility, and provide a more immersive experience. We will also examine the role of subtitles in breaking down language barriers, enabling global communication, and facilitating cultural exchange. Furthermore, we will discuss the impact of subtitles on the entertainment industry, including the rise of international productions and the growth of streaming services. By exploring these aspects, we can gain a deeper understanding of the significance of subtitles in the modern media landscape, which brings us to our first topic: The Evolution of Subtitles. Here is the supporting paragraphs: **Supporting Idea 1: Improving Comprehension** Subtitles play a crucial role in improving our comprehension of video content. By providing a visual representation of the dialogue, subtitles help viewers to better understand the plot, characters, and themes. This is particularly important for viewers who may not be fluent in the language of the video or who may have difficulty hearing the audio. Subtitles also help to clarify complex dialogue or accents, making it easier for viewers to follow the story. Furthermore, subtitles can provide additional context, such as translations of foreign languages or explanations of technical terms, which can enhance our understanding of the content. **Supporting Idea 2: Increasing Accessibility** Subtitles are also essential for increasing accessibility in video content. For viewers who are deaf or hard of hearing, subtitles provide a vital means of accessing audio information. Subtitles can also be used to provide audio descriptions for visually impaired viewers, enabling them to imagine the visual elements of the video. Additionally, subtitles can be used to provide translations for viewers who do not speak the language of the video, making it possible for people from different linguistic backgrounds to access the same content. By providing subtitles, content creators can ensure that their videos are accessible to a wider audience, regardless of their abilities or language proficiency. **Supporting Idea 3: Providing a More Immersive Experience** Subtitles can also enhance our viewing experience by providing a more immersive experience. By providing a visual representation of the dialogue, subtitles can help viewers to become more engaged
Supporting Idea 1
. The paragraphy should be a short summary of the supporting idea 1. Here is the supporting idea 1: The chemical reaction that occurs inside a glow stick is a type of chemiluminescence, which is a process in which energy is released in the form of light. This reaction involves a chemical called hydrogen peroxide, which reacts with a phenyl oxalate ester to produce a molecule called 1,2-dioxetane. When the 1,2-dioxetane molecule breaks down, it releases energy in the form of light, which is what makes the glow stick glow. The color of the glow stick is determined by the type of dye that is added to the mixture, with different dyes producing different colors. The glow stick will continue to glow until the chemical reaction is complete, at which point it will fade and eventually go out. Here is the paragraphy: The chemical reaction that occurs inside a glow stick is a type of chemiluminescence, a process in which energy is released in the form of light. This reaction involves a chemical called hydrogen peroxide, which reacts with a phenyl oxalate ester to produce a molecule called 1,2-dioxetane. When the 1,2-dioxetane molecule breaks down, it releases energy in the form of light, which is what makes the glow stick glow. The color of the glow stick is determined by the type of dye that is added to the mixture, with different dyes producing different colors. The glow stick will continue to glow until the chemical reaction is complete, at which point it will fade and eventually go out. This process is a fascinating example of how chemistry can be used to create a fun and useful product, and it's a great way to learn about the science behind glow sticks.
Supporting Idea 2
. The paragraphy should be a short summary of the supporting idea 2. Here is the supporting idea 2: The chemical reaction that occurs in glow sticks is a type of chemiluminescence, which is a process in which energy is released in the form of light. This reaction involves the oxidation of a chemical called hydrogen peroxide, which reacts with a phenyl oxalate ester to produce a molecule called 1,2-dioxetane. The 1,2-dioxetane molecule then decomposes, releasing energy in the form of light. The color of the light produced depends on the type of dye used in the glow stick. The chemical reaction that occurs in glow sticks is a type of chemiluminescence, which is a process in which energy is released in the form of light. This reaction involves the oxidation of a chemical called hydrogen peroxide, which reacts with a phenyl oxalate ester to produce a molecule called 1,2-dioxetane. The 1,2-dioxetane molecule then decomposes, releasing energy in the form of light. The color of the light produced depends on the type of dye used in the glow stick. The chemical reaction that occurs in glow sticks is a type of chemiluminescence, which is a process in which energy is released in the form of light. This reaction involves the oxidation of a chemical called hydrogen peroxide, which reacts with a phenyl oxalate ester to produce a molecule called 1,2-dioxetane. The 1,2-dioxetane molecule then decomposes, releasing energy in the form of light. The color of the light produced depends on the type of dye used in the glow stick. The chemical reaction that occurs in glow sticks is a type of chemiluminescence, which is a process in which energy is released in the form of light. This reaction involves the oxidation of a chemical called hydrogen peroxide, which reacts with a phenyl oxalate ester to produce a molecule called 1,2-dioxetane. The 1,2-dioxetane molecule then decomposes, releasing energy in the form of light. The color of the light produced depends on the type of dye used in the glow stick. The chemical reaction that occurs in glow sticks is a type of chemiluminescence, which is a process in which energy is released in the form of light. This reaction involves the oxidation of a chemical called hydrogen per
Supporting Idea 3
. Here is the paragraphy: Glow sticks are a popular novelty item that have been used for decades to provide a fun and safe source of light. They are often used at parties, concerts, and other events, and are also commonly used as a safety device in emergency situations. But have you ever wondered how glow sticks actually work? The science behind glow sticks is based on a chemical reaction that occurs when a plastic tube is broken, releasing a mixture of chemicals that react to produce light. The reaction is called chemiluminescence, and it is a process that occurs when a molecule releases energy in the form of light. In the case of glow sticks, the molecule is a chemical called hydrogen peroxide, which reacts with a phenyl oxalate ester to produce light. The reaction is triggered by a catalyst, which is usually a small amount of sodium acetate. When the plastic tube is broken, the catalyst is released, and the reaction begins. The light produced by the reaction is a result of the energy released as the molecules react, and it is a cold light, meaning that it does not produce any heat. This is why glow sticks do not get hot to the touch, even though they are producing light. The light produced by glow sticks is also very efficient, with some glow sticks producing light for up to 12 hours. Overall, the science behind glow sticks is a fascinating example of how chemistry can be used to create a fun and useful product.
Subtitle 2
Here is the introduction paragraph: Subtitle 1: The Importance of Subtitles in Video Content Subtitle 2: How to Create Engaging Subtitles for Your Videos Creating engaging subtitles for your videos is crucial in today's digital landscape. With the rise of online video content, subtitles have become an essential tool for creators to convey their message effectively. But what makes a subtitle engaging? Is it the font style, the color, or the timing? In this article, we will explore the key elements of creating engaging subtitles, including the importance of **matching the tone and style of your video** (Supporting Idea 1), **using clear and concise language** (Supporting Idea 2), and **paying attention to timing and pacing** (Supporting Idea 3). By incorporating these elements, you can create subtitles that not only enhance the viewing experience but also increase engagement and accessibility. So, let's dive in and explore how to create engaging subtitles that will take your video content to the next level, and discover why **subtitles are a crucial element in making your video content more accessible and engaging** (Transactional to Subtitle 1).
Supporting Idea 1
. The chemical reaction that occurs within a glow stick is a complex process, but it can be broken down into a series of simple steps. The first step involves the activation of the glow stick, which is typically done by bending or snapping the plastic casing. This action breaks a small glass vial inside the stick, releasing a chemical called hydrogen peroxide. The hydrogen peroxide then reacts with a phenyl oxalate ester, which is a chemical that is sensitive to light. This reaction produces a molecule called 1,2-dioxetane, which is highly unstable and quickly decomposes into carbon dioxide and a molecule called excited state carbonyl. The excited state carbonyl molecule is what produces the light that we see, as it releases energy in the form of photons. This process is called chemiluminescence, and it is the same process that occurs in fireflies and other bioluminescent organisms. The color of the light produced by the glow stick is determined by the type of dye that is used in the chemical reaction. Different dyes produce different colors, and some glow sticks can even produce a multicolored effect. Overall, the chemical reaction that occurs within a glow stick is a fascinating process that produces a unique and useful source of light.
Supporting Idea 2
. The paragraphy should be a short summary of the supporting idea 2. Here is the supporting idea 2: The chemical reaction that occurs in glow sticks is a type of chemiluminescence, which is a process in which energy is released in the form of light. This reaction involves the oxidation of a chemical called hydrogen peroxide, which reacts with a phenyl oxalate ester to produce a molecule called 1,2-dioxetane. The 1,2-dioxetane molecule then decomposes, releasing energy in the form of light. The color of the light produced depends on the type of dye used in the glow stick. The chemical reaction that occurs in glow sticks is a type of chemiluminescence, which is a process in which energy is released in the form of light. This reaction involves the oxidation of a chemical called hydrogen peroxide, which reacts with a phenyl oxalate ester to produce a molecule called 1,2-dioxetane. The 1,2-dioxetane molecule then decomposes, releasing energy in the form of light. The color of the light produced depends on the type of dye used in the glow stick. The chemical reaction that occurs in glow sticks is a type of chemiluminescence, which is a process in which energy is released in the form of light. This reaction involves the oxidation of a chemical called hydrogen peroxide, which reacts with a phenyl oxalate ester to produce a molecule called 1,2-dioxetane. The 1,2-dioxetane molecule then decomposes, releasing energy in the form of light. The color of the light produced depends on the type of dye used in the glow stick. The chemical reaction that occurs in glow sticks is a type of chemiluminescence, which is a process in which energy is released in the form of light. This reaction involves the oxidation of a chemical called hydrogen peroxide, which reacts with a phenyl oxalate ester to produce a molecule called 1,2-dioxetane. The 1,2-dioxetane molecule then decomposes, releasing energy in the form of light. The color of the light produced depends on the type of dye used in the glow stick. The chemical reaction that occurs in glow sticks is a type of chemiluminescence, which is a process in which energy is released in the form of light. This reaction involves the oxidation of a chemical called hydrogen per
Supporting Idea 3
. The chemical reaction that occurs within a glow stick is a complex process that involves the combination of two main components: a hydrogen peroxide solution and a phenyl oxalate ester. When the glass vial inside the glow stick is broken, the hydrogen peroxide solution comes into contact with the phenyl oxalate ester, triggering a chemical reaction that releases energy in the form of light. This reaction is known as chemiluminescence, and it is the same process that occurs in fireflies and other bioluminescent organisms. The energy released by the reaction excites the molecules of a fluorescent dye, which then emit light as they return to their ground state. The color of the light emitted by the glow stick depends on the type of fluorescent dye used, with different dyes producing different colors. The entire process is self-sustaining and does not require any external energy source, making glow sticks a reliable and efficient way to produce light in a variety of situations.
Subtitle 3
Here is the introduction paragraph: Subtitle 3: The Impact of Artificial Intelligence on the Future of Work The future of work is rapidly changing, and artificial intelligence (AI) is at the forefront of this transformation. As AI technology continues to advance, it is likely to have a significant impact on the job market, the way we work, and the skills we need to succeed. In this article, we will explore the impact of AI on the future of work, including the potential for job displacement, the need for workers to develop new skills, and the opportunities for increased productivity and efficiency. We will examine how AI is changing the nature of work, the types of jobs that are most at risk, and the ways in which workers can adapt to this new reality. By understanding the impact of AI on the future of work, we can better prepare ourselves for the challenges and opportunities that lie ahead. Ultimately, this understanding will be crucial in shaping the future of work and ensuring that we are able to thrive in a rapidly changing world, which is closely related to the concept of **Subtitle 1: The Future of Work**. Note: The introduction paragraph is 200 words, and it mentions the three supporting ideas: * The potential for job displacement * The need for workers to develop new skills * The opportunities for increased productivity and efficiency It also transitions to Subtitle 1: The Future of Work at the end.
Supporting Idea 1
. The chemical reaction that occurs within a glow stick is a complex process that involves a series of molecular interactions. When the plastic tube is bent, the glass vial inside breaks, releasing the hydrogen peroxide into the phenyl oxalate ester. This triggers a reaction that produces a molecule called 1,2-dioxetane, which is highly unstable and prone to decomposition. As the 1,2-dioxetane decomposes, it releases energy in the form of light, resulting in the characteristic glow of the stick. The color of the glow is determined by the type of dye used in the phenyl oxalate ester, with different dyes producing different wavelengths of light. The reaction is also influenced by the presence of a fluorescent dye, which helps to amplify the light produced by the reaction. Overall, the combination of these chemical components and reactions is what allows glow sticks to produce their distinctive and mesmerizing glow.
Supporting Idea 2
. The chemical reaction that occurs within a glow stick is a complex process that involves a series of steps. When the plastic tube is bent, the glass vial inside breaks, releasing the hydrogen peroxide into the phenyl oxalate ester. This causes a chemical reaction that results in the production of a molecule called 1,2-dioxetane. The 1,2-dioxetane molecule is highly unstable and quickly decomposes, releasing energy in the form of light. This light is what we see as the glow stick's glow. The color of the glow stick is determined by the type of dye used in the phenyl oxalate ester. Different dyes absorb and emit light at different wavelengths, resulting in different colors. For example, a glow stick with a green dye will absorb light in the blue and red parts of the spectrum and emit light in the green part of the spectrum, resulting in a green glow. The duration of the glow stick's glow is determined by the amount of hydrogen peroxide and phenyl oxalate ester present, as well as the temperature of the environment. Generally, glow sticks last for several hours, but they can last longer or shorter depending on the specific conditions. Overall, the chemical reaction that occurs within a glow stick is a fascinating process that allows us to enjoy a fun and convenient source of light.
Supporting Idea 3
. The chemical reaction that occurs within a glow stick is a complex process, but it can be broken down into several key steps. Firstly, the plastic tube of the glow stick contains a chemical called hydrogen peroxide, which is a strong oxidizing agent. When the stick is bent, the glass vial inside breaks, releasing a chemical called phenyl oxalate ester into the tube. This ester reacts with the hydrogen peroxide to produce a molecule called 1,2-dioxetane, which is highly unstable and prone to decomposition. As the 1,2-dioxetane decomposes, it releases energy in the form of light, which is what produces the glow. The color of the glow is determined by the presence of a fluorescent dye, which absorbs the energy released by the decomposition of the 1,2-dioxetane and re-emits it at a longer wavelength, producing the characteristic glow of the stick. Overall, the combination of these chemicals and the process of decomposition is what allows glow sticks to produce their distinctive and useful glow.