How Many Valence Electrons Does Nitrogen Have
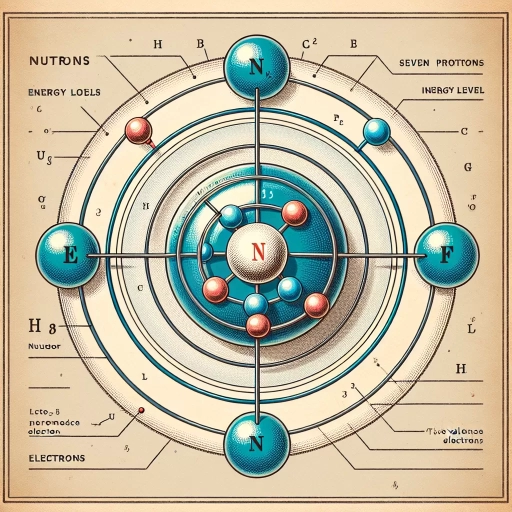
Nitrogen is a fundamental element in our universe, making up a significant portion of the Earth's atmosphere and playing a crucial role in the formation of amino acids, the building blocks of life. As we delve into the world of chemistry, understanding the properties of nitrogen becomes essential. One of the key aspects of nitrogen's chemistry is its valence electrons, which determine its reactivity and ability to form bonds with other elements. But how many valence electrons does nitrogen have? To answer this question, we need to explore the concept of valence electrons, examine nitrogen's atomic structure, and apply this knowledge to determine its valence electrons. In this article, we will first explore the concept of valence electrons, discussing what they are and why they are important in chemistry. By understanding valence electrons, we can then examine nitrogen's atomic structure and determine its valence electrons, providing a comprehensive answer to this fundamental question.
Understanding Valence Electrons
Valence electrons play a crucial role in understanding the chemical properties of elements and their behavior in chemical reactions. To grasp the concept of valence electrons, it is essential to first define what they are and their significance in chemistry. Valence electrons are the electrons present in the outermost energy level of an atom, which participate in chemical bonding and reactions. Understanding the concept of valence electrons is vital as it helps in predicting the chemical properties of elements, such as their reactivity, electronegativity, and ability to form bonds with other elements. In this article, we will delve into the definition of valence electrons, their importance in chemistry, and how they determine the chemical properties of elements. By understanding these concepts, we can gain a deeper insight into the behavior of elements and their interactions with other elements. Let's start by exploring the definition of valence electrons.
Definition of Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom, which participate in chemical bonding. The number of valence electrons an atom has determines its chemical properties and reactivity. In a neutral atom, the number of valence electrons is equal to the number of electrons in the outermost energy level. Valence electrons are typically found in the s and p orbitals of an atom's outermost energy level. The number of valence electrons an atom has can be determined by looking at its electron configuration. For example, the electron configuration of nitrogen is 1s2 2s2 2p3, which means it has five valence electrons. The number of valence electrons an atom has can also be determined by looking at its position in the periodic table. Atoms in the same group (vertical column) of the periodic table have the same number of valence electrons. For example, all the elements in group 15 of the periodic table, including nitrogen, have five valence electrons. The number of valence electrons an atom has is important because it determines the types of chemical bonds it can form. Atoms with a full outer energy level (eight valence electrons) are unreactive, while atoms with a partially filled outer energy level are reactive. The number of valence electrons an atom has also determines its electronegativity, which is a measure of an atom's ability to attract electrons in a covalent bond. Atoms with a high number of valence electrons tend to be more electronegative. In summary, valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding, and the number of valence electrons an atom has determines its chemical properties and reactivity.
Importance of Valence Electrons in Chemistry
Valence electrons play a crucial role in chemistry as they determine the chemical properties of an element. The number of valence electrons in an atom defines its reactivity, the types of bonds it can form, and its overall chemical behavior. Elements with a full outer energy level, such as noble gases, are unreactive due to their stable electron configuration. On the other hand, elements with partially filled outer energy levels, like nitrogen, are highly reactive and tend to form bonds with other atoms to achieve a stable configuration. The valence electrons of an atom also influence its electronegativity, which is the ability of an atom to attract electrons in a covalent bond. Elements with high electronegativity, such as oxygen and fluorine, tend to pull electrons towards themselves, while elements with low electronegativity, like metals, tend to lose electrons. Understanding the valence electrons of an element is essential in predicting its chemical behavior and reactivity, which is critical in various fields, including chemistry, materials science, and pharmacology. By knowing the number of valence electrons in an atom, chemists can predict the types of bonds it can form, its reactivity, and its potential applications. For instance, nitrogen's five valence electrons make it an essential component of amino acids, which are the building blocks of proteins. In conclusion, valence electrons are a fundamental concept in chemistry, and their importance cannot be overstated. They determine the chemical properties of an element, influence its reactivity, and play a crucial role in predicting its chemical behavior. As such, understanding valence electrons is essential for anyone studying chemistry or working in fields related to chemistry.
How Valence Electrons Determine Chemical Properties
Valence electrons play a crucial role in determining the chemical properties of an element. The number of valence electrons in an atom's outermost energy level influences its reactivity, electronegativity, and ability to form bonds with other atoms. Elements with a full outer energy level, such as noble gases, are unreactive due to their stable electron configuration. In contrast, elements with partially filled outer energy levels, like metals and nonmetals, are more reactive and tend to gain or lose electrons to achieve a stable configuration. The number of valence electrons also affects an element's electronegativity, which is its ability to attract electrons in a covalent bond. Elements with a high number of valence electrons, such as fluorine and oxygen, are highly electronegative and tend to pull electrons towards themselves, while elements with a low number of valence electrons, like cesium and francium, are less electronegative and tend to lose electrons. Furthermore, the number of valence electrons determines an element's ability to form bonds with other atoms. Elements with a high number of valence electrons can form multiple bonds, while elements with a low number of valence electrons can only form single bonds. For example, carbon, with four valence electrons, can form four single bonds or two double bonds, while nitrogen, with five valence electrons, can form three single bonds or one triple bond. In summary, the number of valence electrons in an atom's outermost energy level plays a significant role in determining its chemical properties, including reactivity, electronegativity, and bonding ability.
Nitrogen's Atomic Structure
Nitrogen is a chemical element with the symbol N and atomic number 7. It is a colorless, odorless, tasteless, and highly unreactive gas that makes up about 78% of the Earth's atmosphere. Understanding the atomic structure of nitrogen is crucial in various fields, including chemistry, physics, and biology. In this article, we will delve into the atomic structure of nitrogen, exploring its atomic number and mass number, electron configuration, and the number of protons, neutrons, and electrons it contains. By examining these aspects, we can gain a deeper understanding of nitrogen's properties and behavior. Let's start by looking at nitrogen's atomic number and mass number, which provide the foundation for understanding its atomic structure.
Nitrogen's Atomic Number and Mass Number
Nitrogen's atomic number is 7, which means that a neutral nitrogen atom has 7 protons in its atomic nucleus. The mass number of nitrogen is typically 14, which is the sum of the number of protons and neutrons in the nucleus. However, it's worth noting that nitrogen has two stable isotopes, nitrogen-14 and nitrogen-15, with the latter having one more neutron than the former. The atomic mass of nitrogen, which takes into account the naturally occurring isotopes and their relative abundance, is 14.007 u (unified atomic mass units). This value is a weighted average of the masses of the two isotopes, with nitrogen-14 making up about 99.6% of natural nitrogen and nitrogen-15 making up about 0.4%. Understanding the atomic number and mass number of nitrogen is crucial in chemistry, as it allows us to determine the number of electrons in a neutral atom, which in turn affects the chemical properties and reactivity of nitrogen.
Electron Configuration of Nitrogen
The electron configuration of nitrogen is a crucial aspect of its atomic structure, as it determines the arrangement of electrons in the atom. Nitrogen has an atomic number of 7, which means it has 7 protons in its atomic nucleus. The electron configuration of nitrogen is 1s² 2s² 2p³, indicating that the outermost energy level (valence shell) contains 5 electrons. This configuration is a result of the Aufbau principle and the Pauli Exclusion Principle, which state that electrons occupy the lowest available energy levels and that each orbital can hold a maximum of two electrons with opposite spins. The electron configuration of nitrogen is significant because it influences the chemical properties of the element, such as its reactivity and ability to form bonds with other atoms. In particular, the presence of 5 electrons in the valence shell makes nitrogen highly reactive, as it seeks to gain or share electrons to achieve a stable octet configuration. This reactivity is a key factor in nitrogen's role in many biological and chemical processes, including the formation of amino acids, nucleotides, and other essential biomolecules. Overall, the electron configuration of nitrogen is a fundamental aspect of its atomic structure, and understanding it is essential for grasping the element's chemical behavior and biological significance.
Number of Protons, Neutrons, and Electrons in Nitrogen
Nitrogen has 7 protons, 7 neutrons, and 7 electrons.
Determining Nitrogen's Valence Electrons
Determining nitrogen's valence electrons is crucial in understanding its chemical properties and reactivity. Nitrogen, with its atomic number of 7, is a fundamental element in many biological and chemical processes. To determine its valence electrons, we need to look at its valence shell, which is the outermost energy level of an atom. The valence shell of nitrogen contains a specific number of electrons that play a significant role in its reactivity. In this article, we will explore the valence shell of nitrogen, the number of electrons in its valence shell, and how these electrons affect its reactivity. By understanding these concepts, we can gain a deeper insight into nitrogen's chemical behavior and its importance in various biological and chemical processes. First, let's take a closer look at the valence shell of nitrogen.
Valence Shell of Nitrogen
The valence shell of nitrogen, also known as the outermost energy level, is the region where the valence electrons are located. In the case of nitrogen, the valence shell is the second energy level, which can accommodate a maximum of eight electrons. The valence shell of nitrogen is composed of one s-orbital and three p-orbitals, which are arranged in a specific way to minimize electron-electron repulsion. The s-orbital is spherical in shape and can hold a maximum of two electrons, while the p-orbitals are dumbbell-shaped and can hold a maximum of six electrons. In the ground state of nitrogen, the valence shell is occupied by five electrons, with two electrons in the s-orbital and three electrons in the p-orbitals. This configuration is often represented as 1s² 2s² 2p³, where the superscript numbers indicate the number of electrons in each orbital. The valence shell of nitrogen plays a crucial role in determining its chemical properties, such as its reactivity and ability to form bonds with other elements. Understanding the valence shell of nitrogen is essential for predicting its behavior in different chemical reactions and compounds.
Number of Electrons in Nitrogen's Valence Shell
Nitrogen has five valence electrons. Nitrogen is in the second period of the periodic table and the 15th group. The atomic number of nitrogen is 7. The electron configuration of nitrogen is 1s2 2s2 2p3. The valence electrons of nitrogen are the electrons in the outermost shell, which is the second shell. The second shell has five electrons, which are the valence electrons of nitrogen. Therefore, nitrogen has five valence electrons.
How Nitrogen's Valence Electrons Affect Its Reactivity
Nitrogen's valence electrons play a crucial role in determining its reactivity. With five valence electrons, nitrogen is highly reactive and tends to form covalent bonds with other atoms to achieve a stable electronic configuration. The presence of five valence electrons in nitrogen's outermost energy level makes it an excellent candidate for forming bonds with other elements, such as hydrogen, oxygen, and carbon. Nitrogen's reactivity is also influenced by its electronegativity, which is the ability of an atom to attract electrons towards itself. Nitrogen's electronegativity is relatively high, which means it has a strong tendency to attract electrons and form bonds with other atoms. As a result, nitrogen is highly reactive and forms a wide range of compounds, including ammonia, nitric acid, and nitrogen dioxide. The reactivity of nitrogen is also influenced by its ability to form multiple bonds with other atoms, which allows it to form complex molecules with unique properties. Overall, the valence electrons of nitrogen play a critical role in determining its reactivity and its ability to form a wide range of compounds.